

This first issue of the SPTN Newsletter, INTERACTIONS, introduces the SciencePlus Teachers Network. This Network will link junior high science teachers in Nova Scotia and New Brunswick through a newsletter, summer conferences and small group and regional meetings.
You might wonder why there should be a Network. Muriel Smyth, one of the SciencePlus authors recently spoke of her early years of teaching and her sense of isolation. Junior high science teachers face this same isolation, compounded by having few colleagues in their subject area or lacking a background in Science. Breaking down this isolation is the Network's challenge and its task.
The Atlantic Science Curriculum project, which produced SciencePIus, has existed for over fifteen years, beginning as a collaborative, grassroots activity to improve science teaching in the junior high grades. The project evolved into a writing team that produced a series of textbooks which have become the primary science resource in Nova Scotia and New Brunswick schools. But SciencePlus is not the final step. The members of the project are anxious that the ASCP return full circle to the grassroots. We hope that the SPTN will provide an opportunity for this to happen.
The Network, at this stage, is an Advisory Committee made up of about a dozen teachers using SciencePlus. Members of the ASCP Board of Directors will be working with us. However, the Network will work only if it is more than the people who established it. The Network cannot be just us -- it must be you, too.
And what is the newsletter, INTERACTIONS, going to do? We have many ideas and plans. Each issue will offer a place for comments on science teaching and current issues research, examples of what works and what doesn't in SciencePlus, suggestions for student assessment, useful resources and "letters." Other suggested topics for INTERACTIONS include student work, tear-out activities, "predict-observe-explain" problems and science, technology and Society applications.
This column, THE FIRST WORD will cover several aspects of science teaching. We will look at de-mystifying science teaching theory. [What is constructivism anyway?] We want to consider emerging issues in junior high science such as implications of the STS movement. We will welcome advocacy and points of view, providing a platform for authors and teachers. We are anxious to open up subjects for general discussion and we will invite guest editorials from other regions where SciencePlus is used.
Finally, the success of the Newsletter depends on you. If you have something to say about science in junior high science teaching, SciencePlus as a leading tool, or if you have problems and questions which need to be answered, get in touch. Join us in breaking down the isolation. We welcome and solicit your input for the next INTERACTIONS and encourage you to become a part of the SciencePlus Teachers Network.
Does this sound familiar? Have you had similar experiences and frustrations? Frances goes on to write: "These prior beliefs (alternative frameworks, children's science, misconceptions, preconceptions) are extraordinarily resistant to change. Studies reveal that even older students who have had years of exposure to science teaching retain the views of the world and meanings of words held by much younger children. Science teaching will often change students' ideas but not always in the way that was intended."

How do we as science teaches deal with these alternative frameworks? Although the answers are not easy or clear, one approach is to use P-O-E' s What on earth is a P-O-E? It is the name given to a teaching strategy and stands for Predict-Observe-Explain.
Here's one example:
Predict: Hold a ping-pong ball between finger and thumb of one hand and a golf ball between finger and thumb of the other hand. Hold both balls at arm's length and ask the class "What do you (individually) think will happen when I let go?" Gently encourage individuals to give reasons for their answers. Some will say the golf ball will hit the floor first "because it is heavier". Some wet say both will hit at the same time "because the mass doesn't matter". Using the 'predict'' strategy enables the teacher to obtain some important insights into the students' alternative frameworks.
Observe: Let the balls fall. They seem to hit the floor at the same time.
Explain: This is the opportunity for those who thought the golf ball would hit first to adjust their ways of thinking, i.e. their alternative frameworks.
(By the way the golf bail actually hits the floor a fraction of a second before the ping-pong ball. How do you explain that? Look elsewhere in this issue for a suggestion!!)
This teaching strategy is tailor-made for use with SciencePlus. Do you have some P-O-E' s you use and would like to share? Tell us about them -- we'd like to include examples on a regular basis as well as address the issue of coping with alternative frameworks in upcoming issues of INTERACTIONS.
Force meters made from luggage cords could be a hazard.
FLOATING AND SINKING
Don't make your own overflow cans - life is too short!
ELECTRIC CURRENT SP3
Generator (p 150) needs a major demo - why not take apart as bicycle generator? Constructing a motor (p 163) -- got five periods to spare!
PARTICLES SP3
Sublimation with iodine is dangerous (p 352)
Need plastic rods? Someone we know uses ½" plexiglass, cut into 3 cm strips.
CHEMICAL CHANGE SP2
Brainteaser # 17 (p 133) works well as an actual activity.
SOLUTIONS SP1
Kids love chromatography. To tie in with the forgery story, have them test 2 black inks of different makes. Surprising how colorful "black" can be.
Fill the cup with drinking water and ask a student to assist you to fill the test-tubes halfway with water. When the water is poured into # 2, it will turn pink; you can look bewildered, but continue till # 4 has been filled (it will also turn pink). At this stage, you can stop the student and decide you will continue by yourself, as something is obviously going wrong. The student sits down and you pour the contents of all 4 tubes back into the cup.
Now fill test tubes 1, 2, 3, 4 & 5 (all are pink except # 5). Completely exasperated, you pour all 5 tubes back and fill all six tubes. #6 stays pink, while the rest are clear. If you wish, remove the pink colour in # 6 by blowing into the test tube through a straw.
If this doesn't peak their curiosity, check their pulses!

See the diagram for the set-up. (You may use "liquid ammonia" from your local grocery, instead of ammonium hydroxide.) The phenolphthalein turns red in the presence of a base so the students observe a bright pink liquid below and a colorless liquid in the upper flask. As the ammonia gas begins to diffuse into the upper flask, the same pink colour is observed starting to form on top of the filter paper and rising slowly through the liquid into the upper flask. They are asked to write down their observations and to make inferences. These initial ideas can tell you a lot about how students think; i.e. "the top liquid must be thicker because it doesn't come through the paper"; "there must be a vacuum which is sucking the red liquid up"; the bottom liquid must be hot because heat rises .
After discussion of their inferences and the evidence which led them to these conclusions, you may give them some additional information; i.e. show them how phenolphthalein behaves with a base and then allow them to revise their earlier inferences, thus reinforcing the notion that the more information we have, the better the inferences we can make.
Examples of test questions
Each issue will contain examples of test questions. variety is important. Short essays, multiple choice, word usage, completions, corrections, graphing, answering by illustration will all be featured. We have chosen a few examples of questions from units usually studied at this time of year in grades 7 to 9. The questions can be adapted as well, if you wish. Answers are provided where appropriate.
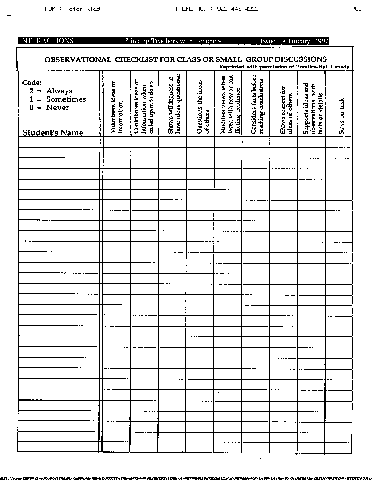
Next, you will find a model (Blackline Master in the printed version) giving a checklist for observing group discussions. It has been adapted from Making the Grade (Prentice-Hall). Modify it for your own needs. Do you have forms you find especially helpful? Share them with other teachers through INTERACTIONS.
Answer: Inertia is the tendency of a body at rest to remain at rest and for a body in motion to remain in motion. When a car is moving, the bodies of the passengers in the car are in motion. If the car comes to an abrupt stop, the passengers have a tendency to keep moving and may even be thrown from the car. Seatbelts overcome inertia by restraining passengers, thus preventing serious injuries in an accident. This is why seatbelts have been required in cars
Answer: Peter used a spring scale to measure the weight of the book and the equal arm balance or platform balance to measure the mass of the book.
a) in front of his skateboard
b) behind his skateboard
c) on his skateboard again
d) to the left of his skateboard
e) to the right of his skateboard

Answer: (c)
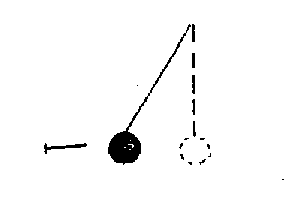
Answer: The copper nail, which is a conductor, cannot be electrically charged when it is rubbed with a wool cloth. Therefore the uncharged pith bail will be unaffected by the copper nail and the diagram should reflect this.
a) design a symbol to represent the two types of centres.
b) Make a card of instructions for each station with clear directions. You may use your imagination and
name the activity. The attached sheet contains blank cards and space for the floor plan.
Each card is already numbered so just add the symbol for the type of activity in the upper
right-hand comer.
Note to Teachers: attach a sheet containing the 10 blank cards needed and space for the floor plan to be drawn.
a) Use the graph to explain what happens to the density of Gas A as it is heated.
b) What happens to the density of Gas B as it is heated?
c) Methane gas has a density of 0.372 grams per litre at 40 C. Which graph represents the gas Methane?
| Temp ( C) | Density (g/L) | |
|---|---|---|
| Gas A | Gas B | |
| 0 | 3.214 | 0.717 |
| 10 | 2.839 | 0.630 |
| 20 | 2.464 | 0.544 |
| 30 | 2.089 | 0.458 |
| 40 | 1.714 | 0.372 |
| 50 | 1.339 | 0.285 |
| 60 | 0.964 | 0.199 |
| 70 | 0.589 | 0.112 |
| 80 | 0.214 | 0.026 |
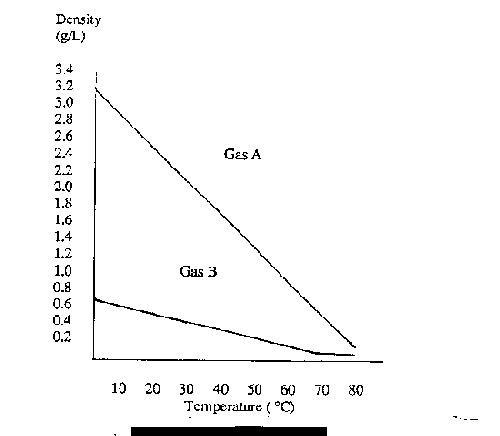
Answer:
(a) As Gas A is heated, its density decreases.
(b) As Gas B is heated, its density decreases.
(c) The Graph of Gas B
Research can take a variety of forms. You are undoubtedly familiar with several. One model is shown here.
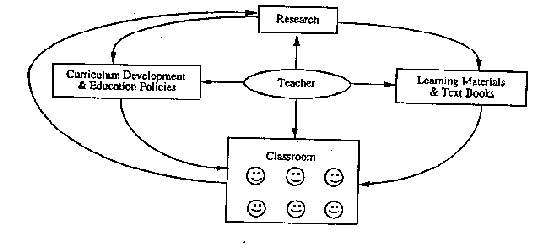
In this case, teachers have input at several stages in the research process and this enables them to play a role in influencing policy formation and curriculum development, a role for which they are often given little credit. This approach also produces a cadre of teachers with experience in education decision-making at many levels, not just in the classroom. Ultimately, this will mean a better education system!
Currently, a similar process specifically aimed at improving science education at the Junior High level is taking place in Nova Scotia and New Brunswick. The project is being conducted by the Atlantic Science Curriculum Project (ASCP), the developers of SciencePlus. It aims to generalize classroom teaching and learning experiences for the purpose of policy development, particularly in relation to the SciencePlus curriculum. It is both classroom-based and teacher-centred. Participants are science teachers in 25 randomly selected and voluntarily participating schools from New Brunswick and Nova Scotia. As such, it is integral to the purpose of both ASCP and the SciencePlus Teachers Network, i.e. linking teaching, curriculum development and research in science education.
Some of !he questions being addressed by ASCP research are:
You can write: INTERACTIONS with your ideas and requests or you can contact:
Chuck McFadden
Director ASCP Research & Development
Faculty of Education, University of New Brunswick
Fredericton, N.B. E3B 6E3.
Phone: 506-453-4695 If no answer: 506-455-2547
Fax: 506-453-3569
mcfad@unb.ca
We are writing regarding Exploration 3, Mini-Experiment #1 on p 434 of SciencePlus 3 (Pressure ). This experiment won't work! We followed your procedure exactly but the
results were not what was expected. The water always came up the tubing. The container was very well sealed so this was not the problem. We have tried this many times with the same results each time Help!
Sincerely,
Class 9A, Rockingham School
Alan Moore, the unit's author, replies:
Oops! You are right. One easily gets a mouthful of water in this activity. The pressure exerted by the air in the flask is greater than the pressure created by sucking on the tube.
Therefore, the water is forced into your mouth.
Can this activity be made to work? Here are 2 suggestions. Try filling the flask completely with water. Can you drink the water through the tube now? Or try using a piece of tubing that is longer than 125 cm. Why should a longer tube make it more difficult to drink from the flask?
After a few seconds, each ball stops accelerating and settles down at its terminal velocity - the downward gravitational force, mg, is balanced by the upward force of air resistance. The terminal velocity for the golf ball is larger because its mass is greater therefore, it reaches the floor slightly before the ping-pong ball
Note: The question of "when" to introduce scientific explanations arises. One suggestion is to do so at the end of a teaching sequence after students have been given the opportunity to make their own frameworks explicit (predict) and to test these against experience (observe, explain).

Nan Armour, Atlantic Co-ordinator
SciencePlus Teachers Network
1331 Brenton Street, Halifax, N.S. B3J 2K5
Phone: (902) 422-5953 FAX: (902) 422-1415
narmour@fox.nstn.ns.ca